| DIMETHYL SULFATE | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 77-78-1 |
|
| EINECS NO. | 201-058-1 | |
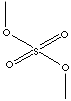
| FORMULA | (CH3O)2SO2 | |
| MOL WT. | 126.13 | |
| H.S. CODE | 2920.90 | |
|
TOXICITY |
Oral rat LD50: 205 mg/kg | |
| SYNONYMS | Methyl Sulfate; Sulfuric acid dimethyl ester; | |
| Dimethyl Sulphate; Dimethylsulfaat (Dutch); Dimetilsolfato (Italian); Dms; Dwumetylowy Siarczan (Polish); Methyle (Sulfate De) (French); Sulfate De Dimethyle (French); Sulfate De Methyle (French); Sulfate Dimethylique (French); Sulfato De Dimetilo (Spanish); Dimethylester Kyseliny Sirove (Czech); Dimethylsulfat (Czech); | ||
|
SMILES |
|
|
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | Clear oily liquid | |
| MELTING POINT | -32 C | |
| BOILING POINT |
189 C | |
| SPECIFIC GRAVITY | 1.333 | |
| SOLUBILITY IN WATER | poor | |
| pH |
| |
| VAPOR DENSITY | 4.3 | |
|
AUTOIGNITION |
495 C | |
|
REFRACTIVE INDEX |
1.3865 | |
|
NFPA RATINGS |
Health: 4; Flammability: 2; Reactivity: 0 | |
| FLASH POINT |
83 C | |
| STABILITY | Stable under ordinary conditions | |
|
APPLICATIONS |
||
| Dimethyl Sulphate is used as a methylation agent in organic synthesis. It is also used as a solvent , stabilizer, sulfonation agent and catalyst. Its end applications include surfactants, pesticides, water treatment chemicals, dyes, flavors, pharmaceuticals, rubber chemicals and photographic chemicals. | ||
| SALES SPECIFICATION | ||
|
APPEARANCE |
Clear oily liquid | |
| ASSAY |
99.0% min | |
| ACIDITY (as H2SO4) |
0.8% max | |
| TRANSPORTATION | ||
| PACKING | 260kgs in drum | |
| HAZARD CLASS | 6.1 8 (Packing Group: I) | |
| UN NO. | 1595 | |
| OTHER INFORMATION | ||
| Hazard Symbols: T+ C, Risk Phrases: 25-26-34-43-45, Safety Phrases: 53-45 | ||
|
GENERAL DESCRIPTION OF ORGANOSULFUR COMPOUNDS |
||
|
Mercaptan: any
of a class of organosulfur compounds is similar to the alcohol and phenol but
containing a sulfur atom in place of the oxygen atom. Compounds containing -SH
as the principal group directly attached to carbon are named 'thiols'. In
substitutive nomenclature their names are formed by adding '-thiol' as a suffix
to the name of the parent compound. When -SH is not the principal group, the
prefix 'mercapto-' is placed before the name of the parent compound to denote an
unsubstituted -SH group. 'thio' is a chemical prefix indicates the replacement
of an oxygen in an acid radical by sulfur with a negative valence of 2. Sulfur
analog of alcohol is called thiol (or mercaptan), and ether analog is called
sulfide.
Sulfate (also spelled sulphates in Euorpe) is any chemical compound related to sulfuric acid, formed by replacing one or both of the hydrogens with a metal or a radical. Sulfite is any salt of sulfurous acid, chemical species H2SO3, which is formed in aqueous solutions of sulfur dioxide. Sulfurous acid is a clear liquid with a strong sulfur aroma. Sulfide is a compound having one or more sulfur atoms in which the sulfur is connected directly to a carbon, metal, or other nonoxygen atom; for example, sodium sulfide, Na2S. Sulfur compounds are used in the synthesis of medicine and chemicals, manufacture of wood, pulp and paper. They are used in winemaking, brewing, food preservation, metallurgy, engraving process, ore flotation, additive in making steel, bleaching, metal treatment and as an analytic reagent. The first chemical contrast of thiols and sulfides with alcohols and ethers is acidity which is important in organic reactions. Thiols are stronger acids than relevant alcohols and phenols. Thiolate conjugate bases are easily formed, and are excellent nucleophiles in SN2 reactions of alkyl halides and tosylates. The nucleophilicity of sulfur is much greater than that of oxygen, resulting in a number of useful electrophilic substitution reaction that are rare by oxygen. For example, sulfides form (with alkyl halides) ternary sulfonium salts, in the same alkylattion of tert-amines quaternary ammonium salts, whereas ternary oxonium salts are prepared only under extream conditions. Without exception, sulfoxides, sulfinate salts and sulfite anion also alkylate on sulfur, despite of the partial negative formal charge on oxygen and partial positive charge on sulfur. The second character is the oxidation states of sulfur. Oxygen has only two oxidation states, whereas sulfur covers from –2 to +6 as follows:
One more sulfur compound's contrast with oxygen analog is in oxidation chemistry. Oxidation of sulfur compounds changes the oxidation state of sulfur rather than carbon, whereas, oxidation of alcohols to aldehydes and ketones changes the oxidation state of carbon not oxygen. Thiol is oxidized to S-S single bond (disufide) which is stronger than O–O bond in peroxide. Disufide forms sulfenyl chlorides (with chlorine in mild condition) or sulfonic acids under harder condition. Oxidation of sulfides with hydrogen peroxide (or peracids) yields sulfoxides and then to sulfones. A certain sulfoxide compound such as dimethyl sulfoxide can be used as an effective oxygen source in the oxidation reaction of primary and secondary alcohols to aldehydes and ketones. DMSO easily is reduced to dimethyl sulfide and water is taken up by the electrophile. oxidation procedure is very mild and tolerates a variety of other functional groups, including those having oxidizable nitrogen and sulfur atoms. Organosulfur compounds are widely used in refineries, steamcrackers, aromatic extraction and petrochemical manufacturing as they acts as hydrotreating catalysts, initial catalyst improvers, sulfur source and catalyst presulfiding. Sulfoxide (R2SO) is any of various organic sulfur compounds having the group -SO (sulfinyl group) whereas sulfone (RSOOR) with the group -SO2 (sulfonyl group). They are derived from oxidation of sulfides. They are widely used as solvent of both extraction and reaction as well as intermediates for the synthesis of textile chemicals and pharmaceuticals and agrochemicals. Dimethyl Sulfoxide is used as an effective extraction solvent and solvent improver for the separation of aromatic compounds (benzene, toluene and xylenes) from aliphatic hydrocarbons, and for fractionation of unsaturated components (olefins and alkynes) from saturated feedstock. It is used as a thermally stable medium for carrying out chemical reactions to make pharmaceuticals, agrochemicals (especially pyrethroid insecticides) paint and coating materials and biocides. Its pharmaceutical grade can be used as a as a local analgesic and anti-inflammatory agent. Sulfolane is used as a extraction as well as a reaction solvent. It is used to separate aromatic hydrocarbons (benzene, toluene and xylenes), It is used to separate n-propyl alcohol and sec-butyl alcohol. It is used to purify natural gas streams and for fractionation of fatty acids into saturated and unsaturated components. It is used as a reaction solvent for the preparation of aromatic sulfonic acids, pyridines, isocyanates and pharmaceuticals. It can also be involved in the halogen exchange process and polymerization process. It is used as a plasticizer and curing agent. Organosulfur compounds have diverse applications in the organic synthesis as organosulfur sources into target organic molecules in the manufacture of pharmaceuticals, adhesives, biocides, agrochemical products, lubricant and fuel additives for high pressure, surfactants, water treatment chemicals, dyes, flavors & fragrances, and photographic chemicals. Sulfonamide is an organic sulfur compounds containing the radical -SO2NH2 (the amides of sulfonic acids). Its molecular structure is similar to p-Aminobenzoic acid (PABA) which is needed in bacteria organisms as a substrate of the enzyme dihydropteroate synthetase for the synthesis of tetrahydrofolic acid (THF). Sulfonamides, derived from chiefly sulfanilamide, are capable of interfering with the metabolic processes in bacteria that require PABA. They act as antimicrobial agents by inhibiting bacterial growth and activity and called sulfa drugs. They are used in the prevention and treatment of bacterial infections, diabetes mellitus, edema, hypertension, and gout. Thiophene, also known as Thiofuran, is a cyclic compound containing four carbon atoms and one sulphur atom in a ring. It is a toxic, flammable, highly reactive, colorless liquid insoluble in water (soluble in alcohol and ether) and melts at 38 C, boils at 84 C. It is used as a solvent and chemical intermediate. Its derivatives are widely used in manufacturing dyes, aroma compounds and pharmaceuticals. They are used as monomers to make condensation copolymers. Thiosulfate is a salt containing the negative ion S2O32-, a analog of the sulfate ion (SO42-) where one of the oxygen (O) atoms has been replaced by a sulfur atom. The sulfur atoms of the thiosulfate ion are not equivalent. Thiosulfate is tetrahedral, and the central sulfur is in the formal oxidation state 6+; and the terminal sulfur is in the formal oxidation state 2-. This species is an important reducing agent. Ammonium Thiosulphate is used as the most common component of photographic fixing agent especially for rapid development. It is used as a nitrogen and sulphur fertilizer. It is used to enhance N utilization efficiency of fertilizers such as urea ammonium nitrate. Sodium Thiosulfate is a white translucent crystals or powder that is common as the pentahydrate form; melting at 48 C; readily soluble in water and oil of turpentine; aqueous solution is slightly alkaline which decompose to sulfate and sulfide in the air. It is a moderate reducing agent. Its major use is as a fixing agent in photography for developing film and the extracting silver from ore. It is used in chrome-tanning leather and in chemical manufacture as a source of sulfide ion. It is also used in paper, textile, water treatment industry and gas purification. Thiocyanate is a salt or ester of thiocyanic acid (HSCN). Aqueous solutions of thiocyanic acid , also called sulfocyanic acid, are very strong acids of the equilibrium mixture of thiocyanic and isothiocyanic. Thiocyanates are bonded through the sulfur(s) with the structure R-S-C≡N or the isomeric R-N=C=S (isothiocyanates). Thiocyanates are bonded through the sulfur(s) which replace for the oxygen (O) atom. Thiocyanates are the sulfur analog of isocyanates. Organic as well as metallic thiocyanates [CuSCN, Ca(SCN)2, NaSCN, KSCN] are very versatile compounds. They have wide range of applications including manufacturing industrial chemicals, pharmaceuticals and pesticides. It is used in freezing solutions, fabric dyeing, electroplating, steel pickling, printing, and corrosion inhibitor against acid gases. It is used in photography industry as a stabilizer or accelerator. Thiourea (also called Thiocarbamide or Sulfourea) is the diamide of thiocarbonic acid that resembles urea but contains sulfur instead of oxygen. In fct, thiourea occurs as the mixture of two tautomers: S=C(NH2)2 ( Thiourea) + HS=CNHNH2 (Isothiourea), accordingly, provides three functional groups (mino, imino, and thiol). Thiourea is a lustrous white crystalline compound; estimated melting point is 170-180 C; soluble in water and in polar organic solvents; insoluble in non-polar solvents. The exact melting point and boiling point are not available since rearrangement to ammonium thiocyanate (NH4SCN) occurs at about 135 C and decomposition occurs. It can be prepared by heating ammonium thiocyanate, or by the addition of hydrogen sulfide to cyanamide. The latter is the more common method. Thiourea is used directly in ore filtering, metal refinery and cleaning, isomerization catalyst (conversion of maleic to fumaric acid) and as an additive in fertilizers (to inhibit the nitrification process), drilling auxiliaries, light-sensitive photocopy paper and explosives. It is used as a fixing agent in photography, as a liquefying agent in animal hide glue, as an insecticide, as a textile-treating agent, and as an intermediate to produce other compounds. Thiourea and its derivatives are versatile intermediates for the synthesis of modified thermosetting resins, thiourea dioxide, dyes, flame retardants, vulcanization accelerators, plant protection agents, pesticides, amino resins, peptizing agents, fungicides, hair preparations, dry cleaning chemicals, corrosion inhibitors and thiazole drugs (e.g., antiseptic, thyrotherapeutic, narcotic, and tuberculostatic agents). Dithiobiurea possesses a wide dipole moment and thus is involved in the forming wide metal chelated complexes as the radioactiv-compound which used in radiopharmaceutical imaging, inhibiting enzyme function, kidney function study and to treat toxic metal poisoning. It is used in co-crystals development used in the field of nonlinear optics to generate new coherent wavelengths. Thioglycolic acid, a simple sulfur group- chained carboxylic acid, is a clear liquid; melts at -16. c, boils at 96 C; soluble in water. It is an useful chemical intermediate in the chemical reactions such as addition, elimination and cyclization. Sulfur group will react with bases, acids, ketones and organic halogen compounds, whereas the carboxylic group will preferentially react In the presence of alcohols or amines. The applications of thioglycolic acid and its derivatives are wide in the fields of of PVC stabilizers, down-hole acidizing, corrosion inhibition in the oil field industry, manufacturing of pharmaceuticals, agrochemicals and dyes, hair care products (waving, hair removal and hair straightening), shrink-resistant treatment of wool, fabric dying, leather processing. Isethionic acid, short chain alkan sulfonate containing hydroxy group, is a water soluble liquid used in the manufacture of mild, biodegradable and high foaming anionic surfactants which provides gentle cleansing and soft skin feel. |
||